DHSC launches call for evidence on inequalities in medical devices and technologies
An independent review by the Department of Health and Social Care (DHSC) has launched a call for evidence to gather insights from experts and organisations, as well as patients and the general public, on the potential racial and gender bias of medical devices.
The call aims to collect evidence and insights regarding potential and known equity issues and solutions to these issues across the entire life cycle of medical devices.
Academics, researchers, health professionals, engineers and device developers, as well as patients and general public, invited to contribute.
Contributions will inform an independent review into potential bias in all types of medical devices and the impact on patients from different socio-demographic groups.
Through commissioning the independent review on equity in medical devices, led by Professor Dame Margaret Whitehead, the government is seeking to tackle disparities in healthcare.
The consultation will support the review by gathering new evidence on if and how medical devices and technologies are biased against patients of different ethnicities, genders and other socio-demographic groups, and what solutions could mitigate these biases.
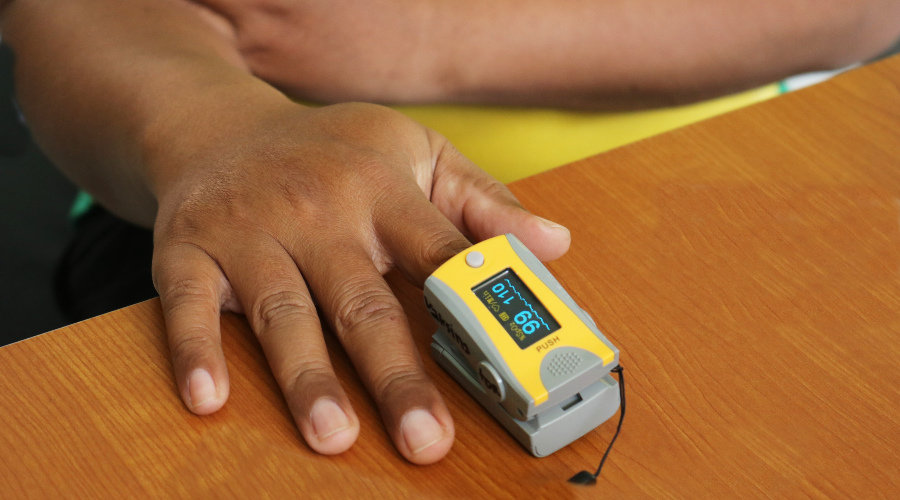
For example, some devices employing infrared light or imaging may not perform as well with patients with darker skin pigmentation, which has not been accounted for in the development and testing of the devices when patients with only a limited range of lighter skin tones were recruited.
The independent review, which the call for evidence will feed into, aims to improve equity in medical devices and help tackle existing healthcare disparities, ensuring people can receive the best-possible care throughout their patient experience regardless of their ethnicity or gender.
Gillian Keegan, Health Minister, said: “I am committed to ensuring all patients receive high-quality, innovative healthcare without discrimination.
“The independent review is part of our vital work to tackle healthcare inequalities, and I invite the industry to share their expertise in the call for evidence so we can ensure medical devices are free of any form of bias.”
Research suggests the way some medical devices are designed and used may be failing to account for differences related to ethnic background, gender or other characteristics – potentially exacerbating existing inequalities in healthcare.
While current UK regulations set out clear expectations on medical devices and technologies, they do not currently include provisions to ensure that medical devices are working equally well for different groups in the population based on their social or demographic characteristics.
The independent review will cover different types of medical devices, including devices enabled by artificial intelligence (AI) used in diagnosing illness and determining therapy pathways, as well as risk-scoring systems using genomics to make decisions about personalised medicine.
It will consider in what ways existing or future regulations could successfully address any biases in medical devices that arise at any stage of their design, development, evaluation, implementation and use.
Patients can be reassured that the NHS is expert in providing the best possible care, and the review is intended to accelerate the process of improving the quality and availability of medical devices to diverse communities.
Professor Dame Margaret Whitehead, chair of the independent review, said: “We aim to establish where and how potential ethnic and other unfair biases may arise in the design and use of medical devices, and what can be done to make improvements.
“We especially encourage patients and members of the public, as well as health, technology and industry experts and researchers to share their views and any evidence concerning medical devices in order to help us tackle inequalities in healthcare.”
With hope to hear from those who work most closely with medical devices such as oxygen measuring devices and infrared scanners and other medical software and hardware, including databases and instructions for medical devices, the call for evidence will remain open for 8 weeks.
The call for evidence applies across a device’s entire life cycle, from evaluation to marketing and implementation, to identify potential biases and solutions at every stage.
The review chair will issue the panel’s report to the Secretary of State for Health and Social Care setting out clear options for consideration by spring 2023 with interim findings expected in winter 2022.