RQM+ shares free search and filter tool for EU Medical Device Regulation with mobility industry
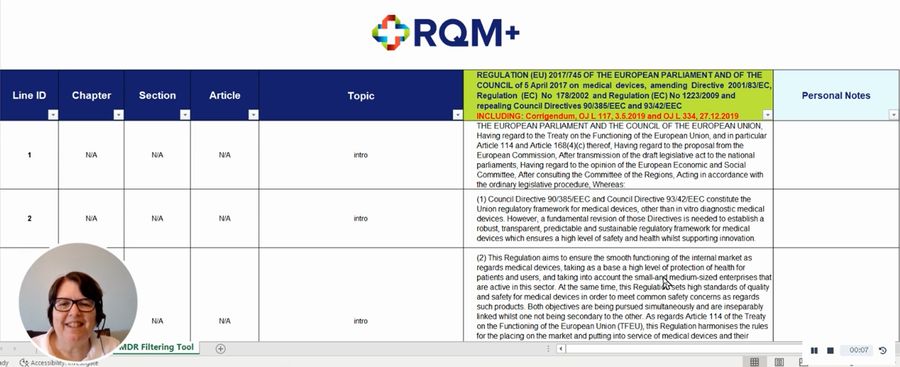
Global regulatory and quality consulting firm RQM+ has created a new tool that allows mobility and access manufacturers to easily navigate the EU Medical Device Regulation (MDR).
The tool enables quick searches by topic, chapter, article and search term, saving regulatory professionals administrative time.
Rather than highlighting or sticky noting a paper copy, users can also save notes on company interpretations of MDR sections and even individual sentences in the tool itself.
The launch comes on the back of an IVDR filtering tool, created to support manufacturers working towards the EU’s upcoming In Vitro Diagnostic Regulation (IVDR).
Both the IVDR and MDR tools are now available for medical device professionals to download and consult for their daily work.
Dr Jaishankar Kutty, vice president of Clinical Services at RQM+, said: “I was recently introduced to this tool after joining RQM+ this February, and my first reaction was: it would’ve been wonderful to have this tool when we waded into the initial MDR reviews at BSI!
“More than any other stakeholder, Notified Bodies need to know the regulation inside-out, and this tool makes it so easy to double check requirements. It’s now sitting on my desktop for easy access and it has been an extremely useful and welcome support so far.”
Nancy Morrison, executive director, Regulatory & Quality Consulting Services, who initially developed these tools to support the RQM+ team commented: “We refer to regulation on a daily basis so we’re well aware of the frustrations of trying to find specific details or to gather all the sections referring to an area of compliance.
“We help our clients to put in place clear and organized processes to facilitate both short- and long-term compliance, and I hope that these tools will also make the work of regulatory professionals a little more straightforward.”


