MHRA issues safety alert for Arjo UK portable patient lift
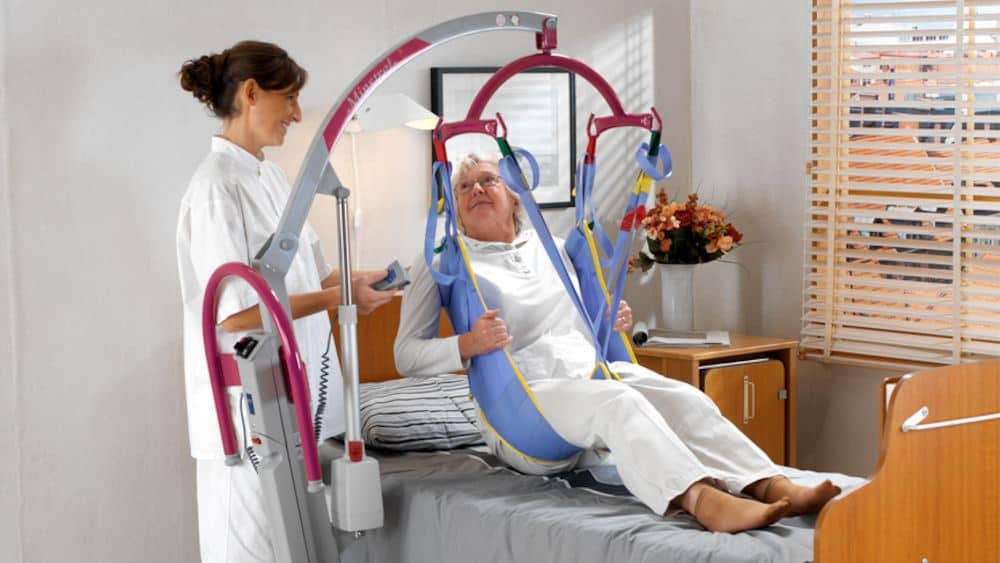
An alert by the Medicines and Healthcare products Regulatory Agency has been issued relating to Arjo UK’s Minstrel passive floor lift, with a risk of the spreader bar detachment from a lift without a scale potentially causing injuries to patients.
Manufactured by ArjoHuntleigh AB, the Minstrel is a versatile, portable resident/patient lift designed for small care facilities, including care homes day centres, hospices, schools and more.
Issued on the 30th January, the alert notice states that there is a risk of the spreader bar detaching from the lift arm during patient transfers, with affected devices being manufactured between January 2008 and March 2010 inclusive, with serial numbers shown in Appendix A of the FSN.
The label found on the Minstrel contains the manufacture date of the device.
According to the Medicines and Healthcare products Regulatory Agency, those responsible for maintaining the Minstrel in care settings should check which version of the spreader bar is fitted using Arjo’s instructions in their Field Safety Notice.
The agency advises those with a device that does need replacing to stop using the patient handling aid immediately, quarantine it and contact Arjo UK.
Following the notice, staff from NHS Trusts, Social Services and establishments registered with the Care Quality Commission (CQC) and establishments registered with OFSTED are being encouraged to inspect their devices to identify if any devices need quarantining.
The manufacturer can be contacted at UKSSUComplaintHandling@arjo.com